Coefficients In A Chemical Equation
Skip to content
Why Is Information technology Of import to Balance Chemical Equations?
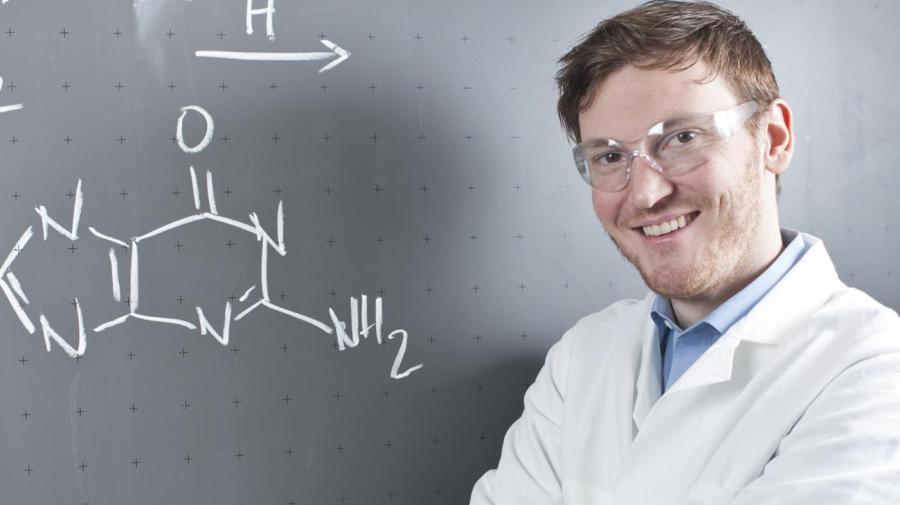
Information technology is important to residuum chemical equations because there must be an equal number of atoms on both sides of the equation to follow the Police of the Conservation of Mass. This chemical law states that in order for the equation to be correct, "An equal quantity of matter exists both before and after the experiment; the quality and quantity of the elements remain precisely the same."
The Police force of Conservation of Mass was discovered by Antoine Laurent Lavoisier in 1789. He discovered that matter cannot either exist created or destroyed. The number on either side of the equation must be exactly the same when the equation is finished as it was when it began.
Equations that are not properly balanced are not correct chemic equations, even if they posses the correct elements and quantities. Unbalanced equations are known as skeletal equations and cannot be used in calculating chemic reactions. Creating a counterbalanced equation rather than using a skeletal equation is very important, equally the number of atoms in a chemic chemical compound always remains the same. Atoms cannot be added to or disappear from an equation. Past making certain the chemical equation is properly counterbalanced, the mass of the chemic chemical compound is correctly preserved.
Coefficients In A Chemical Equation,
Source: https://www.reference.com/science/important-balance-chemical-equations-776a037f89866ac9?utm_content=params%3Ao%3D740005%26ad%3DdirN%26qo%3DserpIndex&ueid=2b42c94c-233a-465b-a47b-f81a8a2a1b84
Posted by: hopperonexped.blogspot.com


0 Response to "Coefficients In A Chemical Equation"
Post a Comment